- A clean 16 ounce plastic soda bottle
- 1/2 cup 20-volume hydrogen peroxide liquid (20-volume is a 6% solution, ask an adult to get this from a beauty supply store or hair salon)
- 1 Tablespoon (one packet) of dry yeast
- 3 Tablespoons of warm water
- Liquid dish washing soap
- Food coloring
- Small cup
- Safety goggles
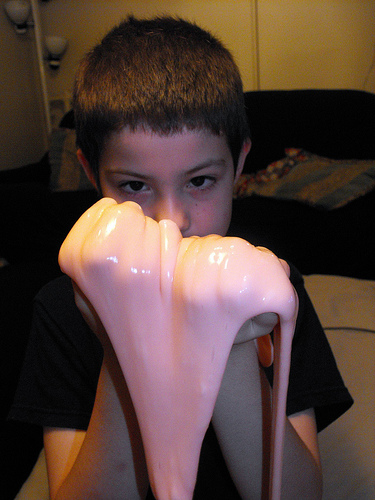
NOTE: As you can see from the picture, foam will overflow from the bottle, so be sure to do this experiment on a washable surface, or place the bottle on a tray.
1. Hydrogen peroxide can irritate skin and eyes, so put on those safety goggles and ask an adult to carefully pour the hydrogen peroxide into the bottle.
2. Add 8 drops of your favorite food coloring into the bottle.
3. Add about 1 tablespoon of liquid dish soap into the bottle and swish the bottle around a bit to mix it.
4. In a separate small cup, combine the warm water and the yeast together and mix for about 30 seconds.
2. Add 8 drops of your favorite food coloring into the bottle.
3. Add about 1 tablespoon of liquid dish soap into the bottle and swish the bottle around a bit to mix it.
4. In a separate small cup, combine the warm water and the yeast together and mix for about 30 seconds.
5. Now the adventure starts! Pour the yeast water mixture into the bottle (a funnel helps here) and watch the foaminess begin!
Foam is awesome! The foam you made is special because each tiny foam bubble is filled with oxygen. The yeast acted as a catalyst (a helper) to remove the oxygen from the hydrogen peroxide. Since it did this very fast, it created lots and lots of bubbles. Did you notice the bottle got warm. Your experiment created a reaction called an Exothermic Reaction - that means it not only created foam, it created heat! The foam produced is just water, soap, and oxygen so you can clean it up with a sponge and pour any extra liquid left in the bottle down the drain.
This experiment is sometimes called "Elephant's Toothpaste" because it looks like toothpaste coming out of a tube, but don't get the foam in your mouth!
The project above is a DEMONSTRATION. To make it a true experiment, you can try to answer these questions:
1. Does the amount of yeast change the amount of foam produced?
2. Does the experiment work as well if you add the dry yeast without mixing it with water?
3. Does the size of the bottle affect the amount of foam produced?
2. Does the experiment work as well if you add the dry yeast without mixing it with water?
3. Does the size of the bottle affect the amount of foam produced?